Initial volume of the balloon =
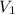 = 348 mL
= 348 mL
Initial temperature of the balloon
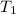 =
=
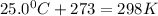
Final volume of the balloon
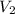 = 322 mL
= 322 mL
Final temperature of the balloon =
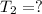
According to Charles law, volume of an ideal gas is directly proportional to the temperature at constant pressure.
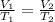
On plugging in the values,
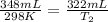
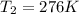
Therefore, the temperature of the freezer is 276 K