Osmotic pressure can be calculated using the following equation:
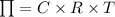
Here,
C representated concentration
R represented gas constant
T represented temperature
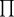 representated osmotic pressure
representated osmotic pressure
R=0.0821 atm L mol ⁻¹
T = 25 + 273 = 298 K
C=
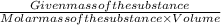
Given mass of the substance= 6.00 g
Volume= 1 L
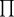 =0.75 atm
=0.75 atm
Putting all the values in the equation:
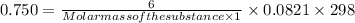
Molar mass of the substance= 195 g mol⁻¹.