Step-by-step explanation:
Atomic number is defined as the sum of total number of protons present in an atom. Whereas mass number is the sum of total number of both protons and neutrons present in an element.
When an atom is neutral in nature then its number of electrons are the same as its number of protons.
For example, atomic number of copper is 29 so it has 29 electrons.
When this copper atom loses two electrons then it acquires a +2 charge and results in the formation of a cation which is
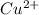 .
.
Thus, we can conclude that when an uncharged copper atom loses two electrons then it results into
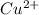 ion.
ion.