Answer:- 0.45L
Solution:- From information, 0.25 L of acetic acid are present in 5 L of a solution. We could calculate the percentage of acetic acid in this solution using the formula:
percent by volume =
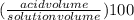
Let's plug in the values in it:
percent by volume =
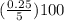
= 5%
Now, it asks to calculate the volume of acetic acid present in 9 L solution with the same percentage. Let's use the same formula again and plug in the values in it:
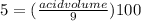
acid volume =
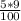
acid volume = 0.45 L
So, 9 L of the solution must contain 0.45 L of acetic acid.