Atomic weight of new element is 342.38 amu. The mass of lighter isotope is 340.91 amu and abundance 68.322 %.
Since, the total abundance is 100% , the abundance of heavier isotope will be (100-68.322) %=31.678%
The formula for atomic weight or average atomic weight of element is as follows:
Average atomic weight= Mass of lighter isotope×% abundance of lighter isotope+Mass of heavier isotope×% abundance of heavier isotope.
Putting the values,
Average atomic weight= 340.91×0.68322+Mass of heavier isotope×0.31678
342.38 amu=232.91+Mass of heavier isotope×0.31678
On rearranging,
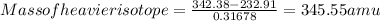
Thus, mass of heavier isotope will be 345.55 amu.