Molality is defined as the ratio of number of moles of solute to the mass of solvent in kilograms.
The formula of molality is:
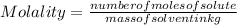 - (1)
- (1)
The formula of density is:
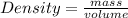 - (2)
- (2)
Consider,
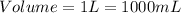
Rearranging the formula (2):
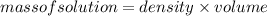
Substituting the values:
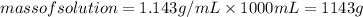
Since, the aqueous solution is 50% by mass that is:
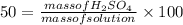
Rearranging the equation:
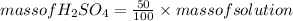
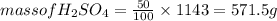
Now, for determining the number of moles of
 :
:
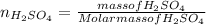
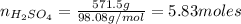
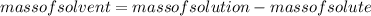
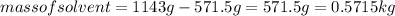
Substituting the values in formula (1):
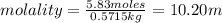
Hence, the molality of the solution is
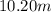 .
.