The molecular mass of calcite is 100.085 (given).
Atomic mass of calcium = 40.078 amu
Percent amount of calcium in calcite =
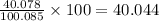 %
%
The total mass of five mineral samples =
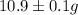 (given)
(given)
So, the mass of one sample =
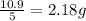
and
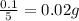
The average mass of calcium in sample =
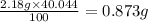
and
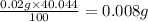
Hence, the average mass of calcium in each sample is
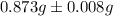 .
.