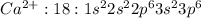Answer:
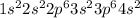 , oxidation state = +2
, oxidation state = +2
Step-by-step explanation:
Electronic configuration represents the total number of electrons that a neutral element contains. We add all the superscripts to know the number of electrons in an atom. The electrons are filled according to Afbau's rule in order of increasing energies.
The electronic configuartion of Calcium with atomic number 20 is :
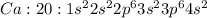
As calcium can easily lose its two valence electrons to attain stability , its oxidation state is +2.