The relationship between pressure and solubility of the gas is given by Henry's law as:
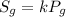
where,
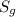 is the solubility of the gas.
is the solubility of the gas.
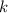 is proportionality constant i.e. Henry's constant.
is proportionality constant i.e. Henry's constant.
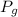 is pressure of the gas.
is pressure of the gas.
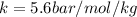 (given)
(given)
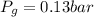 (given)
(given)
Substituting the values,
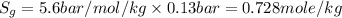
To convert
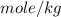 to
to
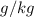 :
:
Molar mass of benzene,
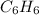 =
=
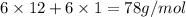
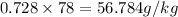
Now for converting into
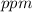 :
:
Since,
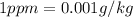
So,
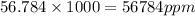 .
.
Hence, the solubility of benzene in water at
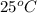 in
in
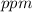 is
is
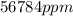 .
.