The volume of sphere can be calculated using the following formula:
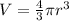
Here, r is radius of the sphere which is 8 cm. Putting the value,
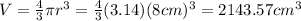
This is equal to the volume of lead, density of lead is
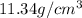 thus, mass of lead can be calculated as follows:
thus, mass of lead can be calculated as follows:
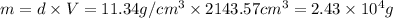
Let the mass of ore be 1 g, 68.5% of galena is obtained by mass, thus, mass of galena obtained will be 0.685 g.
Now, 86.6% of lead is obtained from this gram of galena, thus, mass of lead will be:
m=0.685×0.866=0.5932 g
Therefore, 0.5932 g of lead is obtained from 1 g of ore, if the efficiency is 100%.
For 92.5% efficiency, mass of lead obtained will be:
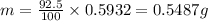
Thus, 1 g of lead obtain from
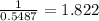 grams of ore.
grams of ore.
Thus,
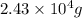 of lead obtain from:
of lead obtain from:
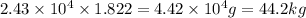
Therefore, mass of ore required to make lead sphere is 44.2 kg.