Answer:- Molecular formula of the compound is
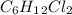 .
.
Solution:- From given information:
C = 46.47%
H = 7.80%
Cl = 45.72%
First of all we find out the empirical formula from given percentages. We divide the given percentages by their respective atomic masses to calculate the moles:
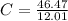 = 3.87
= 3.87
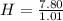 = 7.72
= 7.72
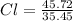 = 1.29
= 1.29
Now, we divide the moles of each by the least one of them. Least one is Cl as it's moles are least as compared to the moles of C and H. So, let's divide the moles of each by 1.29.
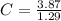 = 3
= 3
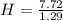 = 6
= 6
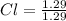 = 1
= 1
So, the empirical formula of the compound is
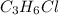 .
.
Empirical formula mass = 3(12.01) + 6(1.01) + 1(35.45)
= 36.03 + 6.06 + 35.45
= 77.54
To calculate the number of formula units we divide molar mass by empirical formula mass.
number of empirical formula units =
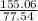
= 2
So, the molecular formula would be two times of empirical formula that is,
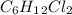 .
.