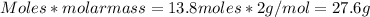Answer:
Mass of H2 = 27.6 g
Step-by-step explanation:
Given:
Moles of CO = 6.50
The balanced reaction is:
8CO + 17H2 → C8H18 + 8H2O
Based on the reaction stoichiometry:
8 moles of CO reacts with 17 moles of H2
Therefore, 6.50 moles of CO would react with:
=
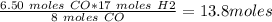
Molar mass of H2 = 2 g/mole
Mass of H2 reacted =