Answer : 70.906 grams of chlorine reacted with hydrogen
Explanation :
Step 1 : Write balanced chemical equation.
The balanced chemical equation for the reaction between hydrogen and chlorine gas is given below.
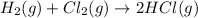
Step 2 : Find moles of H₂ gas.
The moles of H₂ can be found as
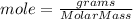
We have 2.0200 g of H₂ and molar mass of H₂ is 2.02 g/mol.
Let us plug in these values to find moles of H₂.
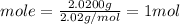
We have 1 mol of H₂.
Step 3 : Find moles of Cl₂ using mole ratio.
The mole ratio of H₂ and Cl₂ is 1 : 1.
The moles of Cl₂ can be calculated as
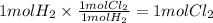
Step 4 : Find grams of Cl₂.
Molar mass of Cl₂ gas is 70.096 g/mol
Mass of Cl₂ =
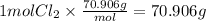
We have 70.906 grams of Cl₂