Mole fraction of both the gases:
Mole fraction of
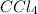 =
=
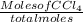
Mole fraction of
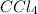 =
=
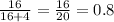
Mole fraction of
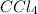 =
=
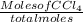
Mole fraction of dioxane =
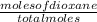
Mole fraction of dioxane =
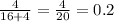
Total pressure of the solution is given by:
mol fraction of
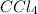
 partial pressure of
partial pressure of
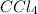 + mol fraction of dioxane
+ mol fraction of dioxane
 partial pressure of dioxane
partial pressure of dioxane
Equating the values in the above equation:
Total pressure of the solution is:
= 0.8
 100 mmHg + 0.2
100 mmHg + 0.2
 38 mmHg
38 mmHg
= 87.6 mmHg