Answer : The molar ratio of C, N and O are 0.05 : 0.01 : 0.1 or 5 : 1 : 10
Explanation :
First we have to calculate the number of moles
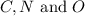 .
.
(1) 2.2 grams of

First we have to calculate the moles of

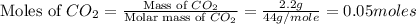
Now we have to calculate the moles of carbon and oxygen.
As, 1 mole of
 contains 1 mole of carbon
contains 1 mole of carbon
So, 0.05 mole of
 contains 0.05 mole of carbon
contains 0.05 mole of carbon
The number of moles of carbon (C) = 0.05 mole
As, 1 mole of
 contains 2 mole of oxygen
contains 2 mole of oxygen
So, 0.05 mole of
 contains
contains
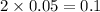 mole of oxygen
mole of oxygen
The number of moles of oxygen (O) = 0.1 mole
(2)
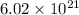 atoms of nitrogen.
atoms of nitrogen.
As,
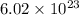 atoms of nitrogen present in 1 mole of nitrogen
atoms of nitrogen present in 1 mole of nitrogen
So,
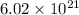 atoms of nitrogen present in
atoms of nitrogen present in
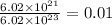 mole of nitrogen
mole of nitrogen
The number of moles of nitrogen (N) = 0.01 mole
From this we conclude that, the molar ratio of C, N and O are:
C : N : O = 0.05 : 0.01 : 0.1
or,
C : N : O = 5 : 1 : 10
Therefore, the molar ratio of C, N and O are 0.05 : 0.01 : 0.1 or 5 : 1 : 10