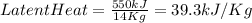Answer:
Specific latent heat = 39.3 kJ/Kg
Step-by-step explanation:
Given:
Mass of the substance = 14 kg
Energy = 550 kJ
Temperature = 262 K
To determine:
The specific latent heat of the substance
Calculation:
The amount of heat (Q) required to change the phase or the physical state of a kilogram (kg) a substance at constant temperature is termed as specific latent heat.
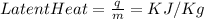
For the given substance: