Answer : The correct option is, (C) 10 M HCl
Explanation :
When 1 mole of HCl dissociates then it gives 1 mole of hydrogen ion
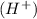 and 1 mole of chloride ion
and 1 mole of chloride ion
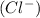 .
.
The balanced chemical reaction is,
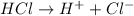
(A) 0.001 M HCl
0.001 M concentration means, 0.001 moles present in 1 liter of solution.
When 0.001 M of HCl dissociates then it gives 0.001 of hydrogen ion
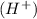 and 0.001 M of chloride ion
and 0.001 M of chloride ion
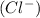 .
.
(B) 0.01 M HCl
0.01 M concentration means, 0.01 moles present in 1 liter of solution.
When 0.01 M of HCl dissociates then it gives 0.01 of hydrogen ion
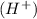 and 0.01 M of chloride ion
and 0.01 M of chloride ion
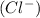 .
.
(C) 10 M HCl
10 M concentration means, 10 moles present in 1 liter of solution.
When 10 M of HCl dissociates then it gives 10 of hydrogen ion
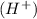 and 10 M of chloride ion
and 10 M of chloride ion
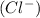 .
.
(D) 0.001 M HCl
0.1 M concentration means, 0.1 moles present in 1 liter of solution.
When 0.1 M of HCl dissociates then it gives 0.1 of hydrogen ion
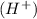 and 0.1 M of chloride ion
and 0.1 M of chloride ion
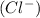 .
.
From this we conclude that, 10 M HCl has the highest concentration of hydrogen ion
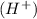 .
.