Answer:
0.5742 moles and grams of potassium manganate are needed to provide 145 grams of potassium nitrate.
Step-by-step explanation:
Mass of potassium nitrate needed = 145 g
Moles of potassium nitrate =
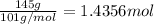
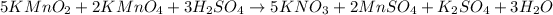
According to reaction, 5 moles of potassium nitrate are obtained from 2 moles of potassium manganate .
Then 1.4356 moles of potassium nitrate will be obtained from:
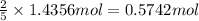
Mass of 0.5742 moles of potassium manganate :
0.5742 mol × 158 g/mol = 90.72 g
0.5742 moles and grams of potassium manganate are needed to provide 145 grams of potassium nitrate.