Answer: The mass of silver nitrate is 137.5 grams.
Step-by-step explanation:
Molarity is defined as the number of moles present in one liter of solution.
Mathematically,
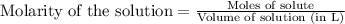
Or,
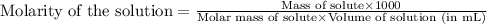
We are given:
Molarity of solution = 5.88 M
Molar mass of silver nitrate = 170 g/mol
Volume of solution = 145 mL
Mass of silver nitrate = ? g
Putting values in above equation, we get:
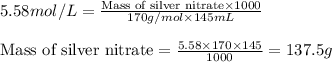
Hence, the mass of silver nitrate is 137.5 grams.