Answer: -
12.41 g
Explanation: -
Mass of CO₂ = 42 g
Molar mass of CO₂ = 12 x 1 + 16 x 2 = 44 g / mol
Number of moles of CO₂ =
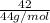
= 0.9545 mol
The balanced chemical equation for this process is
2C₆H₆ + 15O₂ → 12CO₂ + 6H₂O
From the balanced chemical equation we see
12 mol of CO₂ is produced from 2 mol of C₆H₆
0.9545 mol of CO₂ is produced from
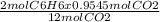
= 0.159 mol of C₆H₆
Molar mass of C₆H₆ = 12 x 6 + 1 x 6 =78 g /mol
Mass of C₆H₆ =Molar mass x Number of moles
= 78 g / mol x 0.159 mol
= 12.41 g