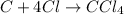Step-by-step explanation:
Carbon has atomic number of 6 with electronic configuration given as:
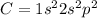
Chlorine has atomic number of 17 with electronic configuration given as:
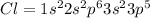
Carbon atom require 4 more electrons to completes its valence shell where as chlorine atom require 1 more electron.
So, in order to gain stable electronic configuration ,1 atom of carbon will form 4 covalent bonds with 4 different chlorine atoms, Hence giving compound named carbon tetrachloride.