Answer:- 8 cylinders are needed to produce the required amount of the gas.
Solution: First we calculate the moles of the gas for it's volume
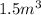 at 200 atm and 150 degree C using ideal gas law equation:
at 200 atm and 150 degree C using ideal gas law equation:
PV = nRT
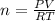
V =
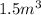 = 1500 L
= 1500 L
P = 200 atm
T = 150 + 273 = 423 K
R =
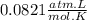
Let's plug in the values in the equation:
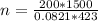
n = 8638.5 mol
Acetylene and nitrogen are 75 mol% and 25 mol% respectively in the cylinder.
So, moles of acetylene =
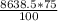 = 6478.9 moles
= 6478.9 moles
moles of nitrogen = 8638.5 - 6478.9 = 2159.6 moles
mass of acetylene = 6478.9*26 = 168451.4 g
mass of nitrogen = 2159.6*28 = 60468.8 g
Total mass of mixture = 168451.4 g + 60468.8 g = 228920.2 g = 228.92 kg
number of cylinders =
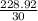 = 7.63 = 8
= 7.63 = 8
So, 8 cylinders are needed.