Answer:
49.8 grams of KI is dissolved in 1 L of solution have a molarity = 0.3M
Step-by-step explanation:
We need the molar mass of KI, we can find the molar mass with the atomic weight of potassium and iodo. We can find this information using the periodic table of the elements.
Atomic weight of
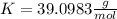
Atomic weight of
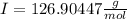
Adding the atomic weights of potassium and iodo, we can obtain the molar mass of KI:
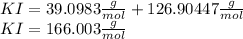
To find the molarity we need the moles of KI in 49.8 g of KI
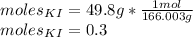
The molarity formula is:
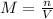
Where:
n= moles of KI
V= volume
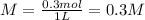
The molarity is 0.3 M