Answer : 1.297 g carbon dioxide will dissolve at 790 mm Hg
Explanation :
According to Henry's law, the amount of gas dissolved in water at a given temperature is directly proportional to its vapor pressure .
In equation format the law can be written as
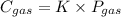
Here C is the concentration of the gas
P is the partial pressure of the gas
K is known as Henry's constant which is temperature dependent.
At 2 different partial pressure values, the equation can be modified as follows
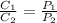
Let us calculate C₁
C₁ = amount of gas in grams / L
C₁ = 1.240 g / 1.01 L
C₁ = 1.228 g/L
P₁ = 755 mm Hg
P₂ = 790 mm Hg
C₂ = ?
Let us plug in the values in Henry's equation
1.228 g L⁻¹ / C₂ = 755 mm Hg / 790 mm Hg
1.228 g L⁻¹ / C₂ = 0.956
C₂ = 1.228 g L⁻¹ / 0.956
C₂ = 1.285 g L⁻¹
The concentration of carbon dioxide at 790 mm Hg is 1.285 g/L
We have 1.01 L water.
Therefore the amount of gas dissolved = 1.01 L x 1.285 g/L = 1.297 g
1.297 g carbon dioxide will dissolve at 790 mm Hg in 1.01 L water