Answer:
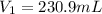
Step-by-step explanation:
Hello,
This is about a dilution exercise which could be mathematically solved by taking into account that during the dilution process the moles are conserved:
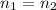
Now, as the moles could be expressed in term of molarity an volume one defines:
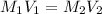
Then, we're asked to report
 as it is the volume we need to take from the inital 2.75M solution of NaOH, thus:
as it is the volume we need to take from the inital 2.75M solution of NaOH, thus:
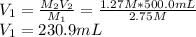
Best regards.