Step-by-step explanation:
A strong base is a base that completely dissociates into ions when dissolved in an aqueous solution.
Upon dissociation a base gives hydroxide or
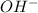 ions. This, means that when we dissolve a base in a solution then we will get more number of hydroxide ions.
ions. This, means that when we dissolve a base in a solution then we will get more number of hydroxide ions.
This will result in decrease in number of hydrogen ions into the solution.
Also,
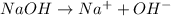
so,
![K_(b) = ([Na^(+)][OH^(-)])/(NaOH)](https://img.qammunity.org/2019/formulas/chemistry/high-school/jy0b63thyy7uiasnt0qqplmj1a3iimljfh.png)
Therefore, we can see that more is the concentration of hydroxide ions more will be the value of
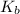 .
.
Thus, we can conclude that following are the characteristics of a strong base.