Answer:
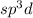
Step-by-step explanation:
Hello,
The type of hybrid orbitals used by the tellurium could be found via the following formula:
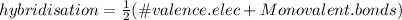
In this case, tellurium has six valance electrons and four monovalent bonds (those four fluorines), thus:
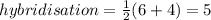
Such result, indicates a
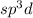 hybridisation which accounts for one ‘s’, three ‘p’ and one ‘d’ orbitals of almost equal energy which give five identical and degenerated hybrid orbitals arranged in trigonal bipyramidal symmetry.
hybridisation which accounts for one ‘s’, three ‘p’ and one ‘d’ orbitals of almost equal energy which give five identical and degenerated hybrid orbitals arranged in trigonal bipyramidal symmetry.
Best regards.