Answer: C) 12.40
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2019/formulas/physics/high-school/iidawris7irvi0bu33z0a9xjzagtaknk6o.png)
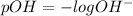
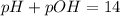
Given:
![[H^+]=0.025 M](https://img.qammunity.org/2019/formulas/chemistry/high-school/nuu3dw8d4rubiknjf84u8d8toue9ze4sjp.png)
![pH=-log[0.025]](https://img.qammunity.org/2019/formulas/chemistry/high-school/cynijvcttfpgy0v3w0wr3et23jgzm60tra.png)
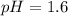
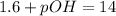
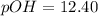
Thus pOH of the solution is 12.40