Answer 1)
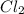
+

----> 2 HCl
This is a pure
chemical reaction that is taking place.
In this reaction the two chemical species which are Chlorine and Hydrogen undergo addition reaction and form two moles of hydrochloric acid. No new species is formed except the combination of reactants which gives the product.
Answer 2) H + H ----> He + n
This is a nuclear reaction.
As the reactant species is undergoing a reaction which is resulting in formation of new species completely different than the elements in reactants.
Also there is a neutron which is generated after the reaction this is clear indication of nuclear fusion reaction. Hence, it can be called as nuclear reaction.