Answer:
The concentration of the HF solution: M = 4.25 M
Step-by-step explanation:
Molarity, denoted by M, is the molar concentration of a given solution. It is the ratio of number of moles of solute and the total volume of the solution in L.
Molarity of a solution is given by,
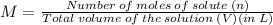
and,
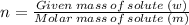
Given: Total volume of solution: V = 2 × 10² mL = 2 × 10² × 10⁻³ L = 0.2 L (∵ 1 mL = 10⁻³ L)
Given mass of solute (HF): w = 17 g, Molar mass of solute (HF): m = 20 g/mol
Molarity of HF solution: M = ?
So the number of moles of solute (HF):
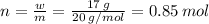
Therefore, the Molarity of the HF solution:
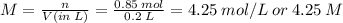
Therefore, the concentration of the HF solution: M = 4.25 M