Answer:
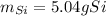
Step-by-step explanation:
Hello,
In this case, we use the Avogadro's number and the atomic mass of silicon to find the grams that are present in that amount of atoms by using the following atoms-mole-mass relationship:
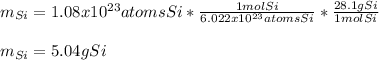
That is why in mole of silicon there are 6.022x10^(23) atoms of silicon as well as 28.1 g of silicon.
Best regards.