Answer : For any exothermic dissolving process of chemical reactions the signs will be as follows ;
Answer 1) Change in enthalpy delta H - In case of exothermic reactions which undergoes heat dissipation which means heat is always given out so
the final enthalpy should be less than the initial enthalpy.
also since H (enthalpy) =
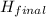 -
-
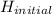 (
(
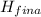 <
<
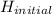 ),
),
So, here
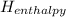 should always be negative.
should always be negative.
Answer 2) Change in entropy delta S - For any reaction entropy is the measurement for change in disorderedness of the reaction. So if we consider in comparison of a lump of crystal to be dissolved in water with a powdered crystal to be dissolved in water; the dissolution of powder will be fast as compared to lump. This means that more the disorderedness more fast the reaction will be. Hence, entropy change should be positive.
Answer 3) Change in free energy delta G - For finding the effect of delta G in exothermic reactions we need to use Gibbs free energy equation:
G = H - TS
Where G is free energy, H is enthalpy, T is the temperature (in Kelvins), and S is entropy.
If we consider H is negative, and entropy is positive, and assume that T is positive , which would mean that the term "TS" should be a positive value always and H should be negative always in exothermic reactions.
so, if we plugging the signs of these entities we get G = (-) - (+) = ( - )
Hence, we can conclude that the free energy of this process should be negative.
Answer 4) Temperature Change (T) - Temperature has to be more than zero K for an exothermic reaction, as the kelvin scale stops at 0.
So if temperature is considered at 0 k then it has to be positive.
If we substitute the value of T as 0 in Gibb's free energy equation we find that,
G = H - 0 S, So G = H. This shows that when the temperature increases more larger TS value would be increased and the TS value would turn more negative.
We can conclude that if we increase the reaction temperature then the G will also be negative favoring an exothermic reaction.
In all we should consider,
H should be negative,
S should be positive,
G should be negative and
T should be positive.