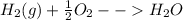Answer:
the law of conservation of mass
Step-by-step explanation:
According to law of conservation of mass we cannot create or destroy matter. During a chemical reaction the matter amount remains the same. The sum of mass of reactants undergoing a reaction will be equal to some of mass of products formed during the reaction.
Now in order to illustrate the same we have to balance a chemical reaction in terms of number of atoms of each elements present in the reactant side and product side.
For example:
If we are writing a reaction of formation of water from hydrogen and oxygen then we have to write it like: