Answer:
3.62 moles
Step-by-step explanation:
Sodium nitride (Na₃N) will decompose as shown in the balanced equation below
2Na₃N → 6Na + N₂
The molar mass of sodium nitride in the solution is
Mass of Na above is (2 ₓ 3 ₓ 23) = 138 g
Mass of N above is (2 ₓ 14) = 28 g
Mass of Na₃N = 138g + 28g = 166g
From the equation above, we can deduce that 166g of Na₃N will produce 6 moles of sodium. Hence 100g of Na₃N will produce X moles of sodium where X is unknown.
∴ 166g of Na₃N → 6 moles of Na
100g of Na₃N → X moles of Na
When the above is cross multiplied,
X =
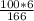
X = 3.62 moles
3.62 moles of sodium (Na) will be formed when 100g of Na₃N decomposes