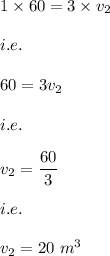Answer:
The new volume that the gas occupy is:
20 cubic meters
Explanation:
Boyle’s law states that the volume of a gas varies inversely with applied pressure.
This means that there exist a constant k such that:
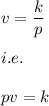
where v is the volume of the gas and is the pressure applied.
This means that if firstly the volume and pressure exerted are:
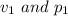 respectively.
respectively.
and afterwards the pressure applied and the volume of the gas is:
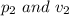 respectively.
respectively.
Then by the inverse relation we have:
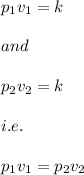
From the data in the question we have:
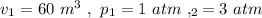
Hence, we have from equation (1)