Answer:
B. 204 amu
Step-by-step explanation:
Given:
Isotopes of Thallium and % abundances
Tl-203 : 29.5%
Tl-205: 70.5%
Formula:
Relative atomic mass =
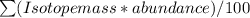
It is given by the summation of the product of all the isotope masses and their % abundances
For Thallium:
Relative atomic mass =
![[203*29.5 + 205*70.5]/100 = 204.41 amu](https://img.qammunity.org/2019/formulas/chemistry/high-school/kdmrnlejo7pyi8hxt47j33dj0uqbdcun72.png)
Since the number of significant digits is 3, the relative atomic mass = 204 amu