Hello!
Data:
Vo (initial volume) = 237.54 mLV (final volume) = ?To (initial temperature) = 45 ºC (to Kelvin)
T (final temperature) = 65 ºC (to Kelvin)
Note: In gas studies the Kelvin scale (K) is used
To (initial temperature) = TC + 273
To (initial temperature) = 45 + 273
To (initial temperature) = 318T (final temperature) = TC + 273
T (final temperature) = 65 + 273
T (final temperature) = 338We have an isobaric transformation, that is, its pressure remains constant, if it increases the temperature the volume also increases, applying the data to the formula, we have:
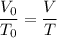
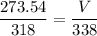
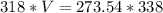
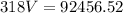
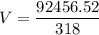
 I hope this helps. =)
I hope this helps. =)