Answer: Therefore, the final volume of the gas will be 3 ml.
Explanation: Combined gas law is the combination of Boyle's law, Charles's law and Gay-Lussac's law.
The combined gas equation is,
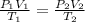
where,
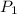 = initial pressure of gas = 1 atm
= initial pressure of gas = 1 atm
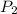 = final pressure of gas = 10 atm
= final pressure of gas = 10 atm
 = initial volume of gas = 20 ml
= initial volume of gas = 20 ml
 = final volume of gas = ?
= final volume of gas = ?
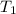 = initial temperature of gas =
= initial temperature of gas =
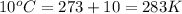
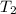 = final temperature of gas =
= final temperature of gas =
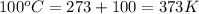
Now put all the given values in the above equation, we get the final pressure of gas.
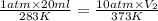
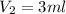
The answer must contain the same number of significant digits as contained in the least precise term.
Therefore, the final volume of the gas will be 3 ml.