Answer : The correct option is "The heat lost by the metal will be equal to heat gained by the water"
Explanation :
In the above problem we have 2 systems, metal and water . Let us find the heat for each system.
The heat, Q is calculated using following formula
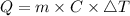
Here m is the mass of the substance.
C is the specific heat of the substance.
ΔT is the change in temperature.
We know that heat flows from a substance which is at higher temperature to the substance at lower temperature.
Since metal is at higher temperature it will lose heat.
The amount of heat lost by metal is
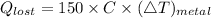
Water is at lower temperature, therefore it will absorb the heat lost by the metal.
The heat gained by water is calculated as
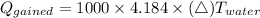
According to thermodynamic principle,
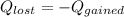
That means heat lost by the metal would be equal to heat gained by the water.