Answer : You can make 4.68 moles of SO₂
Step-by-step explanation:
Step 1 : Write balanced equation.
S₈ can combine with oxygen to form SO₂ gas. The balanced equation for this reaction is written below.
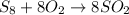
Step 2 : Find moles of S₈
The formula to calculate mol is
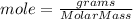
Molar mass of S₈ is 256.5 g/mol
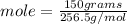
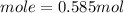
we have 0.585 mol of S₈
Step 3: Use mole ratio to find moles of SO₂
The mole ratio of S₈ and SO₂ can be found using balanced equation which is 1:8
That means 1 mol of S₈ can form 8 moles of SO₂.
Let us use this as a conversion factor to find moles of SO₂
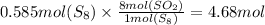
Therefore we have 4.68 moles of SO₂