Average current density of the solution = 1 ampere per square decimeter
We have to find the average current density of the solution in ampere per square meter.
1 decimeter =
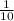
meter
Squaring both sides we get
1 decimeter square =
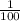
meter square
Current density =
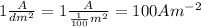
Thus, the density of electrolyte solution is 100 ampere per square meter