The energy required by the excitation of the line is:
ΔE = hν = hc / λ
where:
ΔE = energy difference
h = Planck constant
ν = line frequency
c = speed of light
λ = line wavelength
The energy difference must be supplied by the electron, supposing it transfers all its kinetic energy to excite the line:
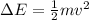
Therefore,
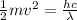
And solving for v we get:
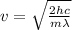
Plugging in numbers (after trasforing into the correct SI units of measurement):
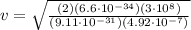
=9.4 · 10⁵ m/s
Hence, the electron must have a speed of
9.4 · 10⁵ m/s in order to excite the 492nm line.