Answer: hexane and 2-methyl pentane
Step-by-step explanation:
The compounds having similar molecular formula but different arrangement of atoms or groups in space are called isomers and the phenomenon is called as isomerism.
Structural isomers are molecules with the same molecular formula but different arrangements of atoms and thus different structures.
Hexane and 2-methyl pentane have the same number of carbon and hydrogen atoms, so they have same molecular formula of
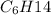 but their structures are different.
but their structures are different.