Answer: Option (a) is the strong acid.
Step-by-step explanation:
Hydrochloric acid is a strong acid and when it is added to water then the following reaction takes place.
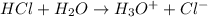
where,
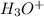 is a conjugate acid and
is a conjugate acid and
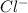 is a conjugate base.
is a conjugate base.
Therefore, when we add HCl into water then it dissociates completely into hydronium ion and chlorine ion.
Thus, we can conclude that a strong acid is disassociated completely, producing more conjugate base.