Answer : Cu²⁺ ion can be most easily reduced.
Explanation :
Let us write the reduction reactions and the associated standard reduction potentials for the given ions.
1)
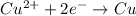
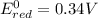
2)
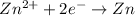
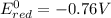
3)
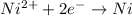
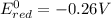
4)
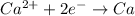
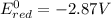
If we look at the above standard reduction potential values, we see that standard reduction potential is highest for Cu²⁺ ion.
Higher the standard reduction potential, greater is the ability to undergo reduction.
Since Cu²⁺ ion has the highest standard reduction potential, it can be most easily reduced.