Answer:
No, potassium chloride will not dissolve completely. Only 28.15 g of potassium chloride will get dissolved.
Step-by-step explanation:
Amount od potassium chloride added to 50 g of boiling water = 250.0 g
The solubility of potassium chloride in boiling water is 56.3 g/100 g water.
Amount of potassium chloride soluble in 100 g of boiling water = 56.3 g
Amount of potassium chloride soluble in 1 g of boiling water =
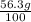
Amount of potassium chloride soluble in 50.0 g of boiling water :
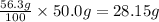
Amount of potassium chloride left undissolved = 250.0 g - 28.15 g = 221.85 g
28.15 g of potassium chloride will dissolve and remaining 221.85 g og potassium chloride will not.