Answer:
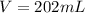
Step-by-step explanation:
Hello!
In this case, considering we have a 5.11-molar solution (5.11 M) that contains 175. g of silver nitrate, we first need to compute the moles of solute as its molar mass is 169.87 g/mol:
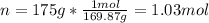
Next, since the definition of molarity is moles over volume:
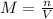
We solve for the volume and plug in the molarity and moles as shown below:
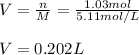
However, as it is needed in milliliters, we convert the L to mL:
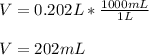
Best regards!