Answer:
e) True, f) False
Step-by-step explanation:
e) Let consider a close system, that is, a system with no mass interactions with surroundings. Then, we get the following expression by the First Law of Thermodynamics:
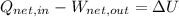 (1)
(1)
Where:
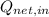 - Net input heat, measured in joules.
- Net input heat, measured in joules.
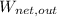 - Net output work, measured in joules.
- Net output work, measured in joules.
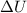 - Change in thermal energy, measured in joules.
- Change in thermal energy, measured in joules.
Please notice that work comprises all kind of work (i.e. mechanical, electric, magnetic), whereas heat comprises all heat interactions including chemical and radioactive phenomena.
If thermal energy is released, then
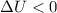 , which is caused by three scenarios:
, which is caused by three scenarios:
(i)
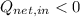 ,
,
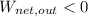 ,
,
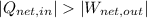
(ii)
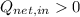 ,
,
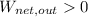 ,
,
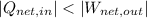
(iii)
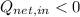 ,
,
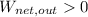
In the case
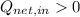 ,
,
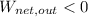 , the thermal energy of the system is increased. Therefore, thermal energy is released during some energy conversions. Answer: True
, the thermal energy of the system is increased. Therefore, thermal energy is released during some energy conversions. Answer: True
f) A liquid solidifies when temperature goes below point of fusion, meaning a realease of heat with no work interactions. That is:
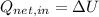 ,
,
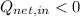 (2)
(2)
If
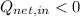 , then
, then
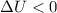 . Then, if a liquid absorbs heat energy, then thermal energy is increase and the liquid does not solidifies. Answer: False.
. Then, if a liquid absorbs heat energy, then thermal energy is increase and the liquid does not solidifies. Answer: False.