Answer: a. same quantities
Explanation: According to avogadro's law, 1 mole of every substance contains avogadro's number
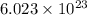 of particles.
of particles.
Molecular weight of
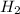 = 2.0 g/mol
= 2.0 g/mol
To calculate the moles, we use the equation:
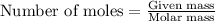
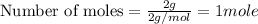
Now 1 mole of
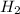 molecule contains =
molecule contains =
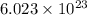 of molecules.
of molecules.
Thus both represent same quantities.