Answer: The correct answer is Option b.
Step-by-step explanation:
Ionic compound is defined as the compound which is formed by the complete transfer of electrons from one atom to another atom. These compounds are usually formed between metals and non-metals.
Covalent compounds are defined as the compounds which are formed by the sharing of electrons between two atoms. These compounds are formed between two non-metals.
The nomenclature of covalent compound is given by:
- The less electronegative element is written first.
- The more electronegative element is written then, and a suffix is added with it. The suffix added is '-ide'.
- If atoms of an element is greater than 1, then prefixes are added which are 'mono' for 1 atom, 'di' for 2 atoms, 'tri' for 3 atoms and so on..
The nomenclature of ionic compounds is given by:
- Positive ion is written first.
- The negative ion is written next and a suffix is added at the end of the negative ion. The suffix added is '-ide'.
For the given compounds:
(I)
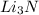 : This is an ionic compound and hence, its name is lithium nitride.
: This is an ionic compound and hence, its name is lithium nitride.
(II)
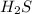 : This is a covalent compound and hence, its name is hydrogen sulfide.
: This is a covalent compound and hence, its name is hydrogen sulfide.
(III)
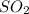 : This is a covalent compound and hence, its name is sulfur dioxide.
: This is a covalent compound and hence, its name is sulfur dioxide.
(IV)
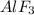 : This is an ionic compound and hence, its name is aluminium fluoride.
: This is an ionic compound and hence, its name is aluminium fluoride.
Hence, the correct answer is Option b.